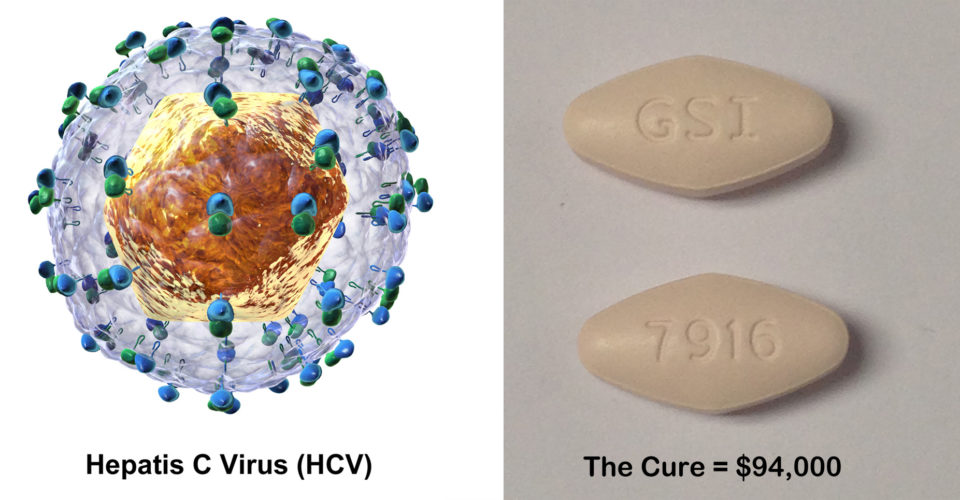
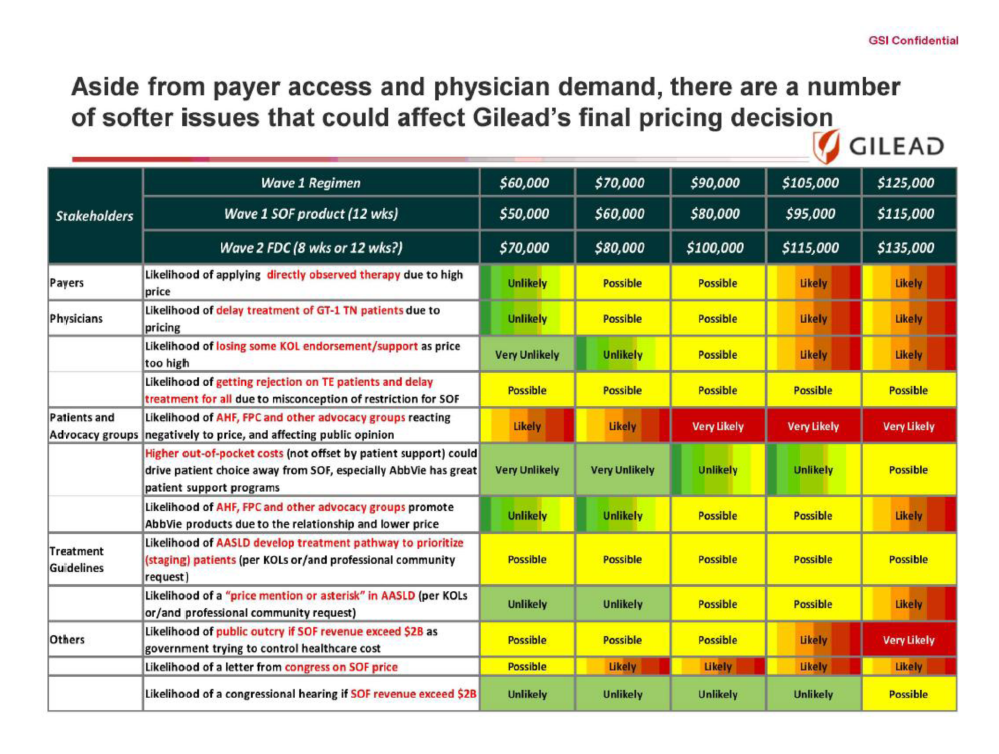
In December, a senate panel investigating the high cost of prescriptions said Gilead used “a calculated scheme for pricing and marketing the drugs based on one goal — maximizing revenues regardless of the human consequence.”
By RICHARD KONTAS
EL NUEVO SOL
I am one of the 3.2 million people in the USA suffering from chronic Hepatitis C (HCV).
The currently available FDA approved treatment(s) boast a 94-97% rate of cure.
OK so what’s the problem you ask?
The medication cost is $94,000 ($1,000/day x 12 weeks) which is precisely the reason that it is not being prescribed.
Yes, modern medicine is really advancing by leaps and bounds; however the pharmaceutical companies (in this case Gilead) are using the pretzel logic of well what can the market bear while comparing answers to the following questions: “likelihood of public outcry if [their] revenue exceeds $2 Billion”, “likelihood of a ‘letter from congress'” or the biggie: “likelihood of a congressional hearing if revenue exceeds $2 Billion.”
Well they pushed it far enough (Gilead 2015 profit $18.11 billion) to warrant a congressional hearing and even after its Full Report nothing has changed regarding the pricing.
Why does this matter?
According to data from the C. Everett Koop Institute at Dartmouth Medical School, the worldwide rate of HCV is 3.3% or roughly 170-200 million people. Hence, it is not as if there are a finite number of patients causing a need to maximize the profit per person that is influencing the price.
So why Gilead, why?
Pure unadulterated GREED; that’s the American way, it certainly isn’t ethical, but it’s legal!
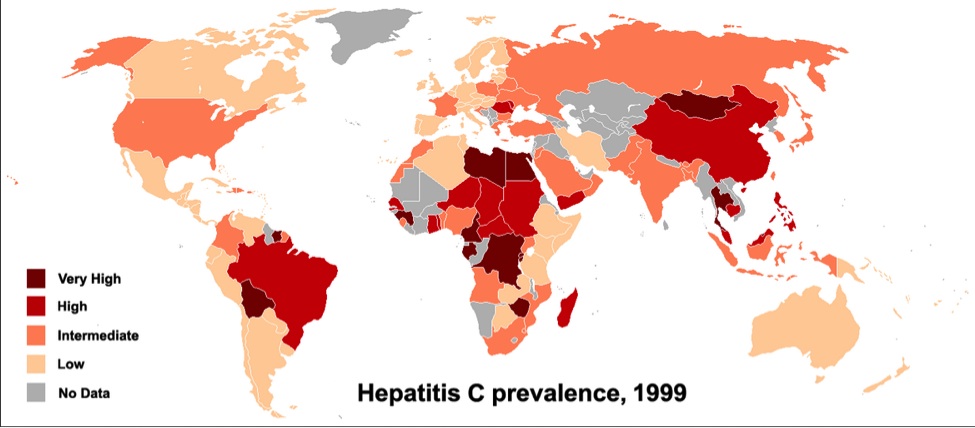
By PhilippN WHO Guide Hepatitis C 2002map based on http://commons.wikimedia.org/wiki/Image:BlankMap‐World.png, CC BY‐SA 3.0, https://commons.wikimedia.org/w/index.php?curid=2127175
A brief background – when first diagnosed in 2002 I was told that the current state of treatment was such that (with severe symptoms) the success rate was only 50% so the thought was to wait; so although I tested positive for HCV I had no symptoms and treatment options would only grow with time.
Then in 2010 I was offered a spot as a likely treatment subject for Peg-interferon/Ribavirin thru LA County — a 48-week regiment with well-known extreme side effects but the only treatment available then. At the time I thought, hey this must be an opportunity and with no insurance I should take advantage of this, so I went in full-bore eyes wide open.
After a year of extreme physical ramifications (so bad that I had to stop treatment for four weeks so my thyroid and my triglyceride level of 1,230 [!!] could be stabilized). I then completed the treatment — during which the virus had become undetectable.
Note that one is not considered ‘cured’ until at least 12 weeks after treatment and still showing a virus free sustained viral response (SVR 12), in my case the virus was back after two weeks. I was somewhat discouraged considering the year it took to get here and the permanent effects on my thyroid.
Moving on I assumed that until new medical options came about there would not be much to do . . . on the bright side they’re always working on new meds and good things were being reported
I heard of these super new fangled meds with ultra hi cure rate 85-90% w/o side effects.
I applied for a clinical trial (this was the original $1,000/day pill), yes, I will be a guinea pig and see what happens. For an idea what’s involved see the Informed Consent Form that one must sign to participate in said trials — in a nutshell it’s for them to cover their butts if things go wrong. Here are the Informed Consent Key Points to be aware of if one were to consider a clinical trial themselves.
After the 3-month screening, tests, lab work, medical history, etc. it was all set to go — it was a randomized trial to compare 8 weeks vs. 12 weeks — I got chosen for the 8-week group and the virus was gone in three days!
The side effects were non-existent and I felt like a new man — birds were chirping, squirrels were prancing about and all was right in the universe. Bear in mind that when you have a chronic disease the quality of life changes aren’t like night and day. They gradually sneak in and so it is not until there is a drastic difference (oh like, let’s say… being cured!) that one becomes aware of them.
In my case the lab work at four weeks post treatment showed the return of the virus — this is called a relapse.
Let’s just say that with this news the birds stopped chirping . . .
In the mean time (this was Dec. 2014) it was suggested that I contact my primary care doctor and request treatment; so far any medical issues I had were treated and virtually everything was free including all prescriptions . . . until now that is.
So my Medi-Cal primary care physician tried at my insistence to refer me to the Olive View liver clinic for treatment – this was back in Dec. 2014 and was told: (HCV Treatment request via Medi-Cal) No referrals are being accepted at this time. When the formulary gets access to new medication you may (or may not) be considered for treatment at that time. Update as of May 11, 2016 still no new meds available.
Take a wild guess about the bird chirping scenario . . . let me help . . . still no chirping, in fact I was starting to think they have permanently changed migration patterns and were gone for good (sarcasm helps fight depression).
In what I consider to be good karma the original clinic that I did the trial with contacted me regarding a new trial that is specifically designed for folks such as myself that are what is called “relapsers.” So it’s back into Guiney pig mode — one of my concerns is that with each failed attempt my body is building a resistance and how many more time can I go thru this — not to mention potentially unknown lasting effects. But hey, once again it’s a crap shoot and I go in with eyes as open as possible.

As of the publication of this article the I am currently in said trial (my third attempt in treatment) and if this does not work then another option would be to move to India under a medical tourism visa – live there for three months and receive the same treatment that is $94,000 in the USA at a cost of less than $1,000.
LA County still has no update on the availability of treatment as the formulary still hasn’t added any medication to its list, while it has been more than a year.
This all comes back to the elephant in the room . . . drug prices are unregulated here in the USA and even the same drugs from the same company elsewhere are available at substantially less cost.
Geez, I hope I get to hear those birds chirping again.
![HCV image by Bruce Blaus (Own work) [CC BY-SA 4.0], via Wikimedia Commons; The Cure image by Richard Kontas](https://elnuevosol.net/wp-content/uploads/2016/05/HCV-Composite1.jpg)
HCV image by Bruce Blaus (Own work) [CC BY-SA 4.0], via Wikimedia Commons; The Cure image by Richard Kontas
Tags: Chronic Hepatitis C HCV Richard Kontas